Synthesis, Identification and Antibacterial Activities of Amino Acid Schiff Base Cu(II) Complexes with Chlorinated Aromatic Moieties
Abstract
:1. Introduction
2. Materials and Methods
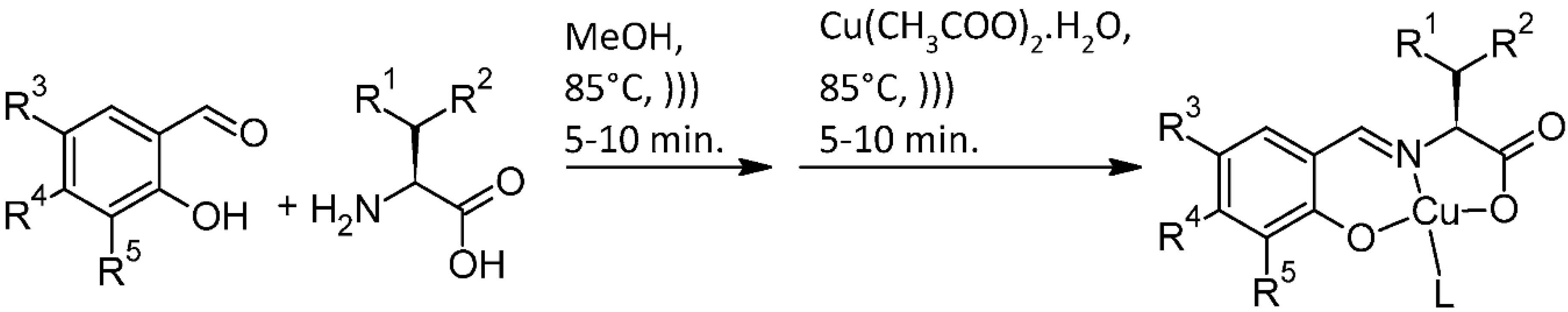
2.1. Compound Syntheses
2.1.1. General Procedures
2.1.2. Physical Measurements
2.1.3. Preparation of C1.2
2.1.4. Preparation of C2.2
2.1.5. Preparation of C3.2
2.1.6. Preparation of C4.2
2.1.7. Preparation of C5.2
2.1.8. Preparation of C6.2
2.1.9. Preparation of C7.2
2.1.10. Preparation of C8.2
2.1.11. Preparation of C9.2
2.1.12. Preparation of C10.2
2.1.13. Preparation of C11.2
2.1.14. Preparation of C12.2
2.2. Antimicrobial Assays
2.3. Antioxidant Assays
2.4. Interaction with E. coli
2.5. Log Po/w Calculation
2.6. Statistical Analysis
3. Results and Discussions
3.1. Chemistry
3.2. Antibacterial Effect
3.3. MIC95 and MIC50 Values
3.4. Antioxidant Activity
3.5. Interaction of Copper Complexes with E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arunadevi, A.; Raman, N. Biological Response of Schiff Base Metal Complexes Incorporating Amino Acids—A Short Review. J. Coord. Chem. 2020, 73, 2095–2116. [Google Scholar] [CrossRef]
- Cimerman, Z.; Galic, N.; Bosner, B. The Schiff Bases of Salicylaldehyde and Aminopyridines as Highly Sensitive Analytical Reagents. Anal. Chim. Acta 1997, 343, 145–153. [Google Scholar] [CrossRef]
- Al-Hussein, M.F.I.; Adam, M.S.S. Catalytic Evaluation of Copper (II) N-Salicylidene-Amino Acid Schiff Base in the Various Catalytic Processes. Appl. Organomet. Chem. 2020, 34, e5598. [Google Scholar] [CrossRef]
- Chakraborty, H.; Paul, N.; Rahman, M.L. Catalytic Activities of Schiff Base Aquocomplexes of Copper(II) towards Hydrolysis of Amino Acid Esters. Transit. Met. Chem. 1994, 19, 524–526. [Google Scholar] [CrossRef]
- Zehra, S.; Roisnel, T.; Arjmand, F. Enantiomeric Amino Acid Schiff Base Copper(II) Complexes as a New Class of RNA-Targeted Metallo-Intercalators: Single X-Ray Crystal Structural Details, Comparative in Vitro DNA/RNA Binding Profile, Cleavage, and Cytotoxicity. ACS Omega 2019, 4, 7691–7705. [Google Scholar] [CrossRef]
- Zuo, J.; Bi, C.; Fan, Y.; Buac, D.; Nardon, C.; Daniel, K.G.; Dou, Q.P. Cellular and Computational Studies of Proteasome Inhibition and Apoptosis Induction in Human Cancer Cells by Amino Acid Schiff Base–Copper Complexes. J. Inorg. Biochem. 2013, 118, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, G.; Ponya Utthra, P.; Raman, N. Exploiting the Biological Efficacy of Benzimidazole Based Schiff Base Complexes with L-Histidine as a Co-Ligand: Combined Molecular Docking, DNA Interaction, Antimicrobial and Cytotoxic Studies. Bioorganic Chem. 2018, 77, 269–279. [Google Scholar] [CrossRef]
- Saikumari, N. Synthesis and Characterization of Amino Acid Schiff Base and Its Copper (II) Complex and Its Antimicrobial Studies. Mater. Today Proc. 2021, 47, 1777–1781. [Google Scholar] [CrossRef]
- Costamagna, J.; Vargas, J.; Latorre, R.; Alvarado, A.; Mena, G. Coordination Compounds of Copper, Nickel and Iron with Schiff Bases Derived from Hydroxynaphthaldehydes and Salicylaldehydes. Coord. Chem. Rev. 1992, 119, 67–88. [Google Scholar] [CrossRef]
- Nakao, Y.; Sakurai, K.; Nakahara, A. Copper (II) Chelates of Schiff Bases Derived from Salicylaldehyde and Various α-Amino Acids. Bull. Chem. Soc. Jpn. 1967, 40, 1536–1538. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The Use of Microwave Ovens for Rapid Organic Synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of Commercial Microwave Ovens to Organic Synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Gedye, R. Microwave-Enhanced Chemistry. Fundamentals, Sample Preparation and Applications Edited by H.M. Kingston (Duquesne University) and Stephen J. Haswell (University of Hull); American Chemical Society: Washington, DC, USA, 1997; p. 772. ISBN 0-8412-3375-6. [Google Scholar]
- Strauss, C.R. Microwave-Assisted Reactions in Organic Synthesis—Are There Any Nonthermal Microwave Effects? Response to the Highlight by N. Kuhnert. Angew. Chem. Int. Ed. 2002, 41, 3589–3591. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric Parameters Relevant to Microwave Dielectric Heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Otani, N.; Furuya, T.; Katsuumi, N.; Haraguchi, T.; Akitsu, T. Synthesis of Amino Acid Derivative Schiff Base Copper(II) Complexes by Microwave and Wet Mechanochemical Methods. J. Indian Chem. Soc. 2021, 98, 100004. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia Coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Kuroda, M.; Yamashita, A.; Hirakawa, H.; Kumano, M.; Morikawa, K.; Higashide, M.; Maruyama, A.; Inose, Y.; Matoba, K.; Toh, H.; et al. Whole Genome Sequence of Staphylococcus Saprophyticus Reveals the Pathogenesis of Uncomplicated Urinary Tract Infection. Proc. Natl. Acad. Sci. USA 2005, 102, 13272–13277. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, P.C.B. Bacillus. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Zhang, R.; Zhang, Y.; Zhang, T.; Xu, M.; Wang, H.; Zhang, S.; Zhang, T.; Zhou, W.; Shi, G. Establishing a MALDI-TOF-TOF-MS Method for Rapid Identification of Three Common Gram-Positive Bacteria (Bacillus Cereus, Listeria Monocytogenes, and Micrococcus Luteus) Associated with Foodborne Diseases. Food Sci. Technol 2022, 42. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.; Shah, D.; Khan, I.; Ahmad, S.; Ali, U.; ur Rahman, A. Synthesis and Antioxidant Activities of Schiff Bases and Their Complexes: An Updated Review. Biointerf. Res. Appl. Chem. 2020, 10, 6936–6963. [Google Scholar]
- Ganji, N.; Rambabu, A.; Vamsikrishna, N.; Daravath, S. Copper (II) Complexes with Isoxazole Schiff Bases: Synthesis, Spectroscopic Investigation, DNA Binding and Nuclease Activities, Antioxidant and Antimicrobial Studies. J. Mol. Struct. 2018, 1173, 173–182. [Google Scholar] [CrossRef]
- Soberanes, Y.; López-Gastélum, K.-A.; Moreno-Urbalejo, J.; Salazar-Medina, A.J.; del Carmen Estrada-Montoya, M.; Sugich-Miranda, R.; Hernandez-Paredes, J.; Gonzalez-Córdova, A.F.; Vallejo-Cordoba, B.; Sotelo-Mundo, R.R.; et al. Tetrameric Copper(II) Metallocyclic Complex Bearing an Amino Acid Derived Schiff Base Ligand: Structure, Catalytic and Antioxidant Activities. Inorg. Chem. Commun. 2018, 94, 139–141. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A. Three ABTS•+ Radical Cation-Based Approaches for the Evaluation of Antioxidant Activity: Fast-and Slow-Reacting Antioxidant Behavior. Chem. Pap. 2018, 72, 1917–1925. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Rensing, C.; Grass, G. Escherichia Coli Mechanisms of Copper Homeostasis in a Changing Environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Duval, R.; Hartemann, P.; Engels-Deutsch, M. Contact Killing and Antimicrobial Properties of Copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Nagashri, K.; Rani, G.A.B. Synthesis, Characterization and Antimicrobial Activities of Copper Complexes Derived from 4-Aminoantipyrine Derivatives. J. Saudi Chem. Soc. 2013, 17, 285–294. [Google Scholar] [CrossRef] [Green Version]


| Entry | Series | Compound | R 1 | R 2 | R 3 | R 4 | R 5 | Yield a |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | C1.1 | CH3 | CH3 | H | H | H | 86% b |
| 2 | C2.1 | OH | H | H | H | H | 65% b | |
| 3 | C3.1 | OH | H | Cl | H | Cl | 49% b | |
| 4 | C4.1 | iPr | H | Cl | H | Cl | 31% b | |
| 5 | C5.1 | H | H | H | H | H | 67% b | |
| 6 | C6.1 | H | H | Cl | H | Cl | 75% b | |
| 7 | C7.1 | OH | CH3 | H | H | H | 86% b | |
| 8 | C8.1 | OH | CH3 | Cl | H | Cl | 78% b | |
| 9 | 2 | C1.2 | iPr | H | H | H | Cl | 55% |
| 10 | C2.2 | iPr | H | H | Cl | H | 33% | |
| 11 | C3.2 | iPr | H | Cl | H | H | 56% | |
| 12 | C4.2 | H | H | H | H | Cl | 65% | |
| 13 | C5.2 | H | H | H | Cl | H | 67% | |
| 14 | C6.2 | H | H | Cl | H | H | 17% | |
| 15 | C7.2 | OH | H | H | H | Cl | 58% | |
| 16 | C8.2 | OH | H | H | Cl | H | 68% | |
| 17 | C9.2 | OH | H | Cl | H | H | 73% | |
| 18 | C10.2 | OH | CH3 | H | H | Cl | 51% | |
| 19 | C11.2 | OH | CH3 | H | Cl | H | 42% | |
| 20 | C12.2 | OH | CH3 | Cl | H | H | 21% |
| Compounds | S. saprophyticus | E. coli | M. luteus | B. subtilis |
|---|---|---|---|---|
| C4.1 | 25/25 | 25/25 | 50/12.5 | >50/50 |
| C6.1 | 12.5/12.5 | 12.5/12.5 | 25/6.25 | >50/50 |
| C2.2 | 50/25 | 6.25/6.25 | 50/12.5 | 6.25/3.12 |
| C8.2 | 50/50 | 50/50 | >50/12.5 | 25/12.5 |
| C9.2 | 50/50 | 50/50 | >50/50 | 25/12.5 |
| C11.2 | 50/50 | 12.5/12.5 | >50/50 | 12.5/12.5 |
| Streptomycin | 25/6.25 | - | - | - |
| Daptomycin | - | - | 7.75/2 | 30/15 |
| Polymyxin B | - | 3.75/1 | - | - |
| C2.2 | C8.2 | C9.2 | C11.2 | AA | |
|---|---|---|---|---|---|
| IC50 (mM) | 0.72 | 0.77 | 0.77 | 0.74 | 0.14 |
| C4.1 | C6.1 | C2.2 | C8.2 | C9.2 | C11.2 | |
|---|---|---|---|---|---|---|
| Absorbance reduction (%) | 60.3 | 34.3 | 34.4 | 11.9 | 2.3 | 82.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, N.; Fayeulle, A.; Nakane, D.; Léonard, E.; Akitsu, T. Synthesis, Identification and Antibacterial Activities of Amino Acid Schiff Base Cu(II) Complexes with Chlorinated Aromatic Moieties. Appl. Microbiol. 2022, 2, 438-448. https://doi.org/10.3390/applmicrobiol2020032
Otani N, Fayeulle A, Nakane D, Léonard E, Akitsu T. Synthesis, Identification and Antibacterial Activities of Amino Acid Schiff Base Cu(II) Complexes with Chlorinated Aromatic Moieties. Applied Microbiology. 2022; 2(2):438-448. https://doi.org/10.3390/applmicrobiol2020032
Chicago/Turabian StyleOtani, Nao, Antoine Fayeulle, Daisuke Nakane, Estelle Léonard, and Takashiro Akitsu. 2022. "Synthesis, Identification and Antibacterial Activities of Amino Acid Schiff Base Cu(II) Complexes with Chlorinated Aromatic Moieties" Applied Microbiology 2, no. 2: 438-448. https://doi.org/10.3390/applmicrobiol2020032
APA StyleOtani, N., Fayeulle, A., Nakane, D., Léonard, E., & Akitsu, T. (2022). Synthesis, Identification and Antibacterial Activities of Amino Acid Schiff Base Cu(II) Complexes with Chlorinated Aromatic Moieties. Applied Microbiology, 2(2), 438-448. https://doi.org/10.3390/applmicrobiol2020032









